10 Functions of Graphene Far-Infrared Rays
Graphene is globally recognized by the scientific community as the best far-infrared radiation material. The heating principle of graphene far-infrared rays is primarily based on
As the preparation technology of graphene becomes more and more mature, graphene is now beginning to be used in various fields, and its strong thermal conductivity and strong electrical conductivity are highly regarded. Can’t help but think of the role of graphene compared to silicon. Will graphene replace silicon?
Table of Contents
Toggle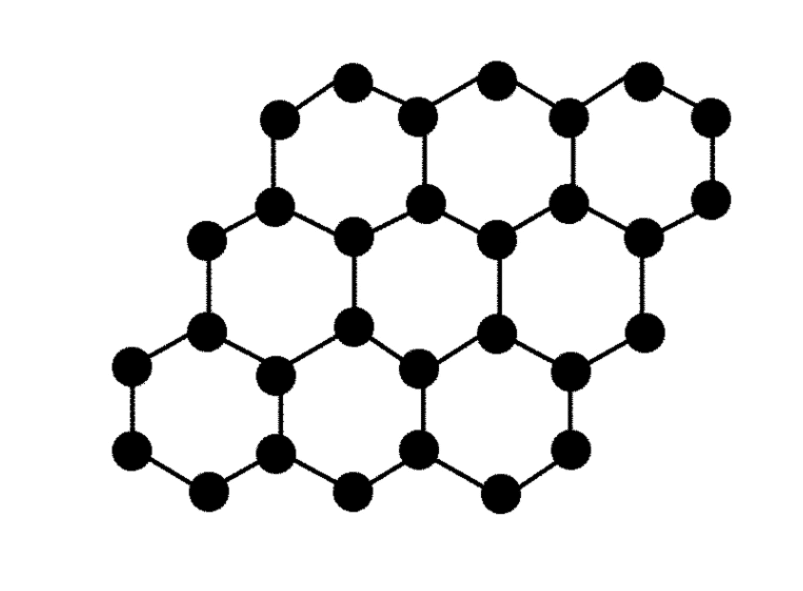
Graphene, with the chemical symbol C, atomic number 6 and relative atomic mass 12.
1. Stability: Graphene’s carbon-carbon bond strength is very high, making it extremely stable at room temperature and pressure. It does not react easily with other chemicals, and this high stability allows graphene to maintain its properties in many harsh environments.
2. Reactivity: Although graphene is very stable under conventional conditions, some of its edges and defective parts can participate in chemical reactions under specific conditions. For example, it can be functionalized by chemical vapor deposition (CVD) or redox methods, adding various functional groups to modulate its properties and applications.
3. Electrical conductivity: Graphene has excellent electrical conductivity, with lower electrical resistance and very high electron mobility compared to metals. This makes graphene a promising application in electronic devices.
1. Mechanical strength: Graphene is one of the strongest known materials, with a Young’s modulus of about 1 TPa and a breaking strength of about 130 GPa, making it hundreds of times stronger than steel, but with a very low density. This makes graphene have great potential for application in high-strength, lightweight composites.
2. Thermal conductivity: Graphene has a very high thermal conductivity, which can theoretically reach 5000 W/m-K, making it one of the best known materials for thermal conductivity. This makes it has a wide range of application prospects in the field of high-efficiency heat dissipation materials and thermal management. For example, graphene electric heating film
3. Optical properties: single-layer graphene has an absorption of visible light of about 2.3%, which, although it may not seem high, in practical applications, the combination of its transparency and conductivity makes it excellent in transparent conductive films and optoelectronic devices.
4. Elasticity and Flexibility: Graphene has good flexibility and elasticity and can be bent or even folded without destroying its structure. This property makes it an important application in flexible electronic devices, wearable devices and other fields.
Graphene is a nanomaterial with a special structure of a single layer of carbon atoms that gives it some incredible properties. Listed below, if there is an error you can contact us to point it out.
Graphene has excellent conductivity, with electron mobility in it far exceeding that of silicon. This means that graphene has significant advantages in high frequency electronics and fast switching.
Graphene is the thinnest and strongest known material. It has a Young’s modulus of up to 1 TPa and is about 200 times stronger than steel. Graphene is also very flexible and can be stretched to 20% of its original length.
Graphene also has a very high thermal conductivity of 5000 W/(m-K), which gives it great potential for heat dissipation and thermal management. Currently graphene sheet tim is used for chip cooling in various high-end cell phone models.
Graphene is almost completely transparent, with an absorption rate of only 2.3%. This makes it a promising material for a wide range of applications in optoelectronic devices and transparent electronics.
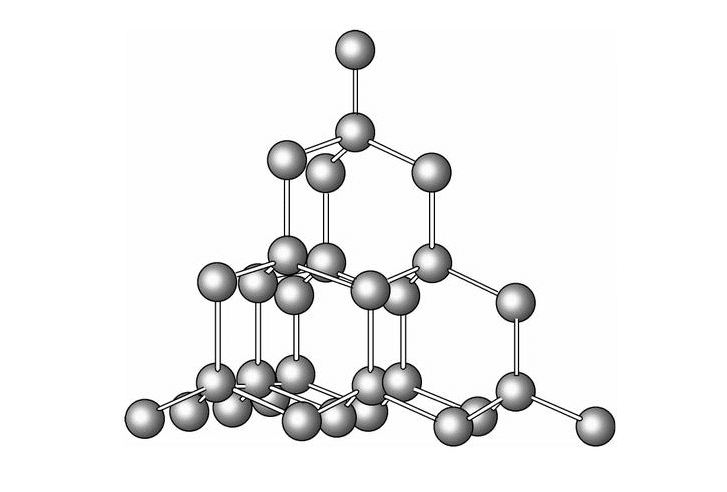
The silicon atom, known by the symbol “Si”, has an atomic number of 14 and a relative atomic mass of 28.
1. Chemical stability: Silicon is relatively stable at room temperature and is resistant to corrosion by most acids, but is highly reactive to hydrofluoric acid (HF) and concentrated alkalis. Silicon in the air will form a thin layer of silicon dioxide (SiO₂) protective film, this layer of oxide film can prevent further oxidation.
2. Oxidizing: Silicon reacts with oxygen to form silicon dioxide (SiO₂), and this oxide film plays a protective role on the surface of silicon. At high temperatures, silicon can react with oxygen to form different types of oxides, such as silicon dioxide (SiO₂) and silicon trioxide (SiO₃), which are widely used in materials science.
3. Reactivity: Silicon is less reactive than metallic elements, but can still react with some non-metallic elements. For example, at high temperatures, silicon can react with nitrogen, chlorine, hydrogen, etc. to produce silicon nitride (Si₃N₄), silicon chloride (SiCl₄) and silane (SiH₄) and other compounds. Silane compounds are widely used in the semiconductor industry.
4. Acid-base reactions: Silicon reacts with concentrated hydrofluoric acid (HF) to produce soluble silicon fluoride (SiF₄); when reacting with strong bases, soluble silicates can be produced, such as sodium silicate (Na₂SiO₃) in sodium hydroxide solution.
Silicon is a semiconducting element with a tetrahedral structure and a diamond-like atomic arrangement. This structure gives silicon its high mechanical strength and hardness. The crystal structure of silicon makes it widely used in electronics and optoelectronics.
the melting point of silicon is 1414 ° C, boiling point of 2900 ° C. These high melting and boiling points make silicon can be stable at high temperatures, widely used in high-temperature environment of the electronic components.
Silicon is a typical semiconductor material. The electrical conductivity of pure silicon is poor, but when doped with different elements (e.g., phosphorus, boron), its electrical conductivity is greatly enhanced, a characteristic that makes silicon important in integrated circuits and semiconductor devices. The electrical conductivity of silicon is relatively low at room temperature, but much higher than insulators.
Silicon has a high thermal conductivity (about 149 W/m-K), which means that it can conduct heat efficiently. Therefore, silicon is widely used in electronic components that require heat dissipation, such as computer chips.
Silicon’s high hardness (7 on the Mohs scale) makes it useful as a wear-resistant material in many industrial applications. However, it is brittle and prone to rupture when subjected to external forces.
Silicon is opaque in the visible range, so it is not usually used in optical applications that require a transparent material, but it has applications in infrared optics (especially long-wave infrared).
Silicon has a density of about 2.33 g/cm³, which is lighter than many metals and alloys, but provides sufficient strength and stability in semiconductor materials.
1. Mature manufacturing process: The processing technology of silicon materials has been developed over decades and is very mature. The processes of silicon wafer fabrication, doping, etching and integrated circuits are very well developed and less costly.
2. Abundant resources: Silicon is one of the most abundant elements in the earth’s crust, and raw materials are easy to obtain and low cost.
3. good semiconductor properties: silicon has good semiconductor properties, easy to control the energy band structure so that it excels in a variety of electronic devices.
4. High degree of integration: silicon integrated circuit technology has been able to realize the high degree of integration of billions of transistors on a single chip, which meets the stringent requirements of modern electronic devices on performance and volume.
Graphene is indeed superior to silicon in many ways, especially in terms of electrical and thermal conductivity and mechanical properties. Graphene has an extremely high electron mobility, theoretically orders of magnitude higher than silicon, and thus has the potential to provide enhanced performance in high-speed, high-frequency electronics. In addition, graphene’s flexibility and strength make it a great potential for flexible electronics, transparent conductive films, and sensors.
The challenge with graphene, however, is its bandgap. Silicon has a moderate bandgap, making it suitable for use as a semiconductor material that can control the flow of electric current well, while graphene is naturally a zero bandgap material, which makes it difficult for it to “switch” the current in traditional semiconductor applications like silicon. Although scientists have been studying the introduction of a bandgap for graphene through the application of electric fields, nanostructure modulation and other methods, these technologies are not yet fully mature, and may involve complex manufacturing processes.
The reason why silicon has been able to dominate in the past decades is not only its material properties, but also the maturity of silicon-based semiconductor technology. The processing of silicon materials has reached a very high level, and the entire industrial chain is very complete, from raw material extraction to wafer production to micron- or even nanometer-scale integrated circuit manufacturing. Moreover, the raw material resources of silicon are abundant and the cost is low.
In contrast, although the preparation technology of graphene has made great progress in theory, a series of technical and economic difficulties need to be overcome in order to industrialize graphene, scale it up and compete with silicon in terms of cost. The production of graphene is still facing problems such as high cost, low yield and unstable quality, which are difficult to be solved in the short term.
Even if the preparation technology of graphene is mature, it may not completely replace silicon. Silicon and graphene each have different advantages and are suitable for different application scenarios. For example:
● Graphene: It is suitable for applications that require extremely high conductivity, transparency, flexibility, etc., such as transparent touch screens, flexible electronic devices, supercapacitors, sensors, optoelectronic devices, etc. Graphene has great potential in these areas, especially in next-generation communication technologies (e.g., 5G, 6G) and high-end technologies such as quantum computing, where it may replace or work in concert with silicon.
● Silicon: Silicon remains the workhorse material in traditional semiconductor applications, especially in digital computing, memory, sensors and most electronic devices. Silicon’s bandgap properties make it very effective in these areas, and silicon’s production processes are well established for large-scale manufacturing.
As a result, graphene and silicon are more likely to complement each other in the future than to be direct substitutes. Graphene may replace silicon in some specific areas, but silicon’s dominance in most traditional electronic devices still cannot be easily surpassed.
A more realistic scenario may be that graphene and silicon are used in combination in future electronic devices with complementary advantages. For example:
● Silicon-graphene composites: Scientists are already working on composites that combine graphene with silicon, a material that can combine the advantages of both, maintaining the semiconducting properties of silicon while taking advantage of graphene’s conductivity, heat dissipation, and other advantages. This composite material is expected to make breakthroughs in high-frequency, high-power, highly integrated electronic devices.
● Silicon-based graphene devices: Silicon can be used as the base material in future integrated circuits, while graphene can be used to improve conductivity, reduce power consumption, or provide higher operating frequencies. For example, in certain high-performance computing and communication chips, graphene could be used as a wire material, thermal management material, etc., while silicon maintains its core semiconductor function.
Nowadays, the preparation technology of graphene powder has been able to realize large-scale and low-cost production in China. It does have the potential to replace silicon in some areas, especially in scenarios requiring extremely high performance or novel applications. However, due to silicon’s technological maturity and cost advantages, silicon will not be completely replaced by graphene in the foreseeable future. Instead, it is more likely that graphene and silicon will complement each other in several areas and work together to drive innovation in electronics.
In short, whether graphene will replace silicon depends on future technological advances, cost reductions, and application needs in different fields. It may not completely replace silicon, but become a powerful complement to silicon, driving the emergence of more efficient and innovative electronic devices and systems.
○ Principles of Chemistry (textbook for chemistry majors) – This book usually gives a detailed description of the properties of the elements.
○ Fundamentals of Materials Science and Engineering – Covers the structure and properties of silicon and other semiconductor materials.
○ Semiconductor Physics and Devices – Specializes in the use of semiconductor materials such as silicon in electronics.
○ For example, Materials Science and Engineering, Journal of Applied Physics, Nature Materials, etc. often cover research and applications of silicon materials.
○ Research papers can be searched on databases such as Google Scholar, PubMed, Scopus, etc. to find literature on the chemical and physical properties of silicon.
○ CRC Handbook of Chemistry and Physics – This book provides physicochemical properties of a large number of chemical elements and materials.
○ ASM Handbook – It mainly explains the physical and chemical properties of materials and is suitable for understanding the specific applications of different materials.
○ For example, Semiconductor Materials: Materials, Processing, and Devices provides insight into the semiconductor properties and applications of silicon.
We produce and develop graphene raw materials and graphene applications, with 50 researchers, and are open to working with you to develop customized products.
Graphene is globally recognized by the scientific community as the best far-infrared radiation material. The heating principle of graphene far-infrared rays is primarily based on
Source: Internet Graphene’s thermal conductivity is a highly researched field, thanks to its exceptional heat conduction properties and potential applications in thermal management. At room
With the passing of 2024 and the arrival of 2025, graphene heating technology is developing faster and faster, and there are more and more people’s
Two Misconceptions About Graphene Heating Film Two Common Misconceptions About Graphene Heating Films: Have You Fallen for Them? Introduction to Graphene Graphene is a revolutionary
Experience the excellent energy-saving underfloor heating brought by new technology
We’ll get back to you in 1 business day.